Ionization Energy Worksheet - Answer Key
Back to the other Periodic Table Trends Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is ionization energy?
The energy required to remove an electron from a gaseous atom/ion.
- General trend on the periodic table:
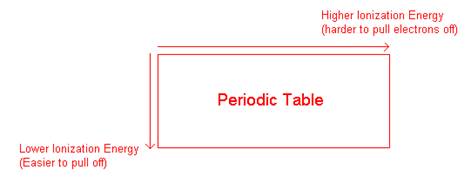
- What can ionization energy tell us about core and valence electrons?
The trend in ionization energies can indicate when we have switched from pulling valence electrons to pulling core electrons.
- Why does phosphorus have a first ionization energy of 1060 kJ/mol and sulfur have a first ionization energy of 1005 kJ/mol?
P: [Ne]3s23p3
S: [Ne]3s23p4
As you can see from the electron configuration of phosphorus and sulfur, the electrons in the phosphorus configuration are fully spread out over the 3p orbitals. In the sulfur, on the other hand, there would be some repulsion between the electrons sharing the orbital. Because of this repulsion is would be easier to pull the sulfur electron out.
- Which configuration would have the highest 1st ionization energy and which would have the lowest 2nd ionization energy?
- 1s22s22p6 – this would have the highest first ionization energy.
- 1s22s22p63s1
- 1s22s22p63s2 – this would have the lowest first ionization energy.
- Which in each set has the smallest 1st ionization energy?
- Ca, Sr, Ba
- N, O, F
- S2-, S, S2+
- To which family would the following element belong?
Group 2
